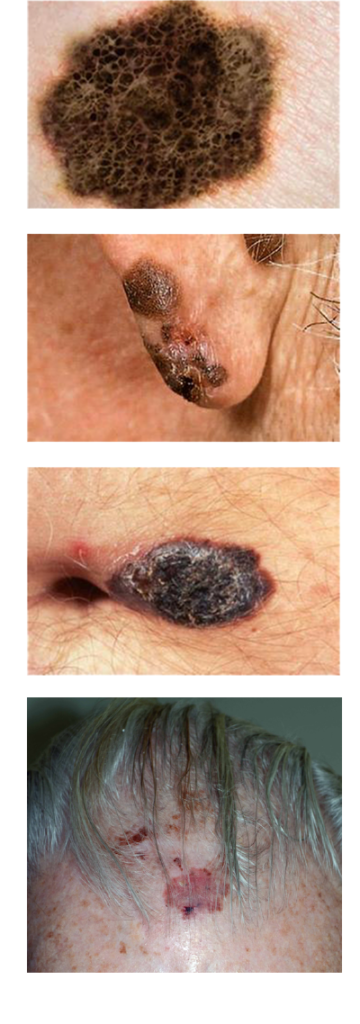

Melanoma is a term to describe all the forms of cancer that occur over the entire span of the body. It is a common misconception that melanoma can only occur on the surface of the skin. However, melanoma can occur inside the body as well, but is more prevalent on the surface of the skin.
In a quick search on the web, the term melanoma can be confusing, and sometimes terrifying. Dr. Jacobsen will be able to explain these issues to you simply and address any other concerns you may have.
It can be terrifying once you hear of a melanoma diagnosis. Despite the shock of a diagnosis, melanoma can be treated with surgery very quickly, and promptly. In most cases be identified and taken care of before the melanoma can spread any further.
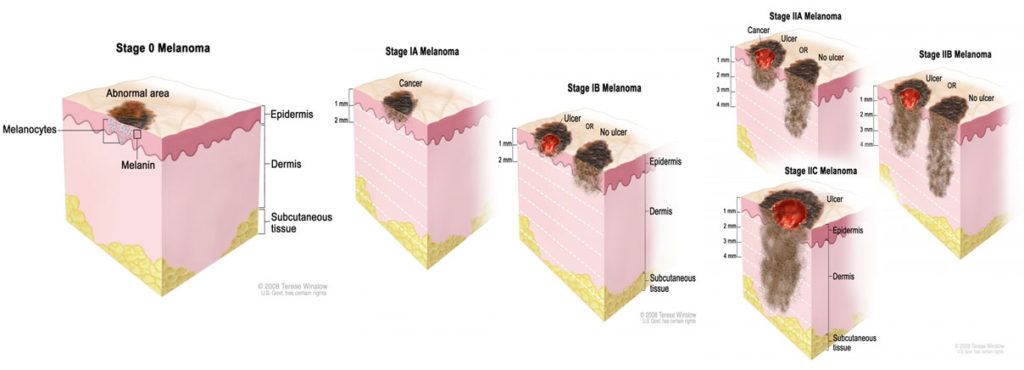
Apart from other types of skin cancers, specific to melanoma, a wider margin of skin must be removed around the sighting to prevent future recurrence. In practice we say the melanoma must be removed with “clear margins”, in its entirety and can be seen and measured under a microscope. In some case the removal of a lymph node from the area may be required. The severity, or curability, of the melanoma is most directly related to its’ thickness in millimeters. Something called “Breslow’s Depth”. Dr. Jacobsen works closely with specialty oncologists to help in the overall care and follow up on your removal.
Once the area containing the melanoma is removed the wound must be closed as perfectly as possible, minimizing the scar and time it takes you to heal. One of the benefits of seeing a doctor who specializes in Cosmetic and Restorative Surgery is the precision and finely done repairs that are possible on your skin and body.
With many years of removing melanoma and professionally repairing the area, Dr. Jacobsen is an experienced melanoma specialist in Phoenix, and deals with it almost every day here at his practice.
Dr. Jacobsen and his staff realize how terribly important their skin is to their patients and how anxious patients may become, naturally, when they are informed that they have melanoma by their doctor or dermatologist. Dr. Jacobsen and his staff work close together to get appointments in as soon as possible and schedule very next day appointments. Having you treated as soon as possible.
When calling or emailing for an appointment, please make sure that you have your pathology report from your dermatologist or family doctor so we can design the best possible treatment for you.
Again, keep in mind the treatment for melanoma is 99.9% surgical. It is very unlikely the patient will need any other treatment except the surgery itself. Nearly every patient will require some sort of surgery to cure them of this terrible disease. The vast majority of patients who undergo surgery for melanoma do very well and it never recurs. It is only in patients with quite advanced melanoma that it tends to reoccur or become a life-threatening disease. Each patient needs a separate evaluation and unique surgical plan. Come see us and we will put your mind at rest, explain everything to you in detail and take excellent care of you.
Call or email us with you skin cancer question and we will help you.
Considering Your Options
With Phoenix consisting of warmer weather and hot sun, having or developing melanoma in Phoenix is not uncommon – and nothing to be ashamed of. Because of
the common occurrences of melanoma in our city, Phoenix Melanoma Surgeons are regarded as some of the best in the world. If you think you have melanoma or think
you’ve developed melanoma, it is important you see a specialist regarding your melanoma. Please feel free to refer to the rest of this page to learn more about
melanoma and what you can do to work towards becoming melanoma-free. Keep in mind surgery is the treatment.
Surgery for Melanoma Skin Cancer
Surgery is 99% of the care needed for melanoma. Surgery is the primary cure for melanoma. All other treatments for melanomas fall after surgical treatment. There are rare exception to this, but these are indeed rare. The proper surgical excision of a melanoma is the most effective treatment possible for your melanoma.
Your Melanoma Pathology Report
The Melanoma Pathology Report is very difficult and technical to read. This can be very frightening, which is why Dr. Jacobsen will comfort and go over all the details
of the report for and with you. Keep in mind the most important factor is the thickness of the melanoma. The melanoma thickness is expressed in millimeters.
Generally, the melanoma the biopsy thickness is added to the melanoma thickness from the secondary removal. Dr. Jacobson will sort all this out with you.
Common Surgical Techniques for Phoenix Melanoma Removal
Wide Excision
Melanoma should be treated with a wide excision. Normally with other skin cancers
just removing a small amount of normal skin on either side of the cancer will be
curative. However, with melanoma it is important to perform a wide excision, or what
is called a “radical excision”, which means that you will need to have 1 cm or 2 cm more of additional skin around your biopsy area of the melanoma to be treated
properly. This requires surgical reconstruction most times, which is why plastic surgeons and not dermatologist perform this treatment. Depending on the part of
the body that is involved it can be difficult to remove the extra tissue, or this wide excision. Particularly if this occurs on the face. Normally, however, melanoma
occurs on the extremities; the trunk or the scalp, but not the face. Melanomas rarely occur on the eyelids or the nose but, occasionally do on the lips.
Depending on the thickness of your melanoma you will need more or less tissue removed. If you have melanoma in “situ”, which is a pre-melanoma you may only need a small amount removed. Local anesthesia used on a small area can be removed in the office. Oftentimes, however, for larger resections this is done under light general anesthesia at the surgery center.
The removed sample is then evaluated by professional pathologists that specialize in melanoma skin tumors to determine that the melanoma is completely removed in its entirety.
Patients always ask if melanomas extend to the bone or not. Not only can melanoma effect the skin to the left and right of the tumor, but can also take particularly deeply down. Up until the muscular layer. Rarely ever touching the muscular layer, nerves, arteries or bones.
As a part of his practice, Dr. Jacobsen will draw on your body part exactly the amount that needs to be removed. The variability in the amount of skin that is removed is dependent on the thickness of the melanoma which Dr. Jacobson can review with you.
Mohs surgery
Mohs surgery is used for skin cancers but is not standardly used for melanoma. The main reason for this is that melanoma cannot be seen easily under the quick staining
and microscopic procedures done in a Mohs surgeon’s office. To accurately see melanoma significant, specialized, staining techniques must be used under a microscope, sometimes taking up to 3 working days for results. The margins of Melanoma cannot be seen accurately with quick frozen sections that are required and done at the same time of the resection. As a result, Mohs surgery is not done for melanoma. Most dermatologists will refer melanoma patients to a plastic surgeon or to a general surgeon.
Amputation
In some situations, amputation can be a tool for cure of melanoma. This is quite rare however. It is only appropriate for quite advanced melanoma of the extremities, or for amputating the finger tips or digits that results from melanoma that has advanced under the fingernail, in the fingertip or toenails. Amputations are rarely required for melanoma.
Lymph node removal
In this operation, the surgeon removes all the lymph nodes in the region near the primary melanoma. For example, if the melanoma is on a leg, the surgeon would
remove the nodes in the groin region on that side of the body, which is where melanoma cells would most likely travel to first.
Once the diagnosis of melanoma is made from the skin biopsy, the doctor will examine the lymph nodes near the melanoma. Depending on the thickness and
location of the melanoma, this may be done by physical exam, or by imaging tests (such as CT or PET scans) to look at nodes that are not near the body surface.
If the nearby lymph nodes are abnormally hard or large, and a fine needle aspiration (FNA) biopsy or excisional biopsy will be able to test for melanoma in a node or
nodes, before a lymph node dissection is usually done.
If the lymph nodes are not enlarged, a sentinel lymph node biopsy may be done, particularly if the melanoma is thicker than 1 mm. (See Tests for Melanoma Skin
Cancer for a description of this procedure.) If the sentinel lymph node does not contain cancer, then there is no need for a lymph node dissection because it’s
unlikely the melanoma has spread to the lymph nodes. If the sentinel lymph node contains cancer cells, removing the remaining lymph nodes in that area with a
lymph node dissection is usually advised. This is called a completion lymph node dissection and is very rarely needed today.
It’s not clear if a lymph node dissection can cure melanomas that have spread to the nodes and is still being studied. Still, some doctors feel it might prolong a patient’s
life and at least avoid the pain that may be caused by cancer growing in these lymph nodes, but less than 4% of patients actually benefit from complete lymph node removal.
A full lymph node dissection can cause some long-term side effects. One of the
most troublesome is called lymphedema. Lymph nodes in the groin or under the arm normally help drain fluid from the limbs. If they are removed, fluid may build up.
This can cause limb swelling, which may or may not go away. If severe enough, it can cause skin problems and an increased risk of infections in the limb. Elastic
stockings or compression sleeves can help some people with this condition. For more information, see our Lymphedema section. Lymphedema, along with the pain
from the surgery itself, is a main reason why lymph node dissection is not done unless the doctor feels it is necessary.
Sentinel lymph node biopsy, however, is unlikely to have the same effect. It’s important to discuss the risks of side effects with your doctor before having either of these procedures.
Postoperative Lymphocele
Some patient’s after the simple lymph node removal develop some fluid under their arm. This is called a lymphocele and can be drained very easily at Dr. Jacobson’s office with a small needle and a syringe. About 50% of patients develop this fluid collection after a sentinel lymph node biopsy for malignant melanoma or after the removal of a lymph node. Dr. Jacobsen will be following along very carefully with you. Some patients require 2 or 3 drainage visits, but most need none at all.
Surgery for Metastatic Melanoma
If melanoma has spread from the skin to other places in your body such as the lungs or brain, the cancer is not likely to be curable by surgery. Even when only 1 or 2
areas of spread are found by imaging tests such as CT or MRI scans, there are likely to be more metastases, too small to be found by these scans.
Surgery for Melanoma Metastases
Surgery is sometimes done in when there are metastases. This is intended in rare circumstances to control the cancer rather than cure it. If a limited number of
metastases are present and can be removed completely, this surgery may help some people live longer. Removing metastases in some areas, such as the central
nervous system, might also help prevent or relieve symptoms and improve a patient’s quality of life. But is very rare that surgery for distant melanoma tumor or
metastasis of melanoma response to surgery. Since immunotherapy now is so effective, surgical procedures for advanced melanoma is rarely necessary.
Treating Melanoma Skin Cancer
Once melanoma has been diagnosed and staged, your oncologist will discuss treatment with you. It’s important that you think carefully about your options. There
are new options that are very effective now that we did not have available for patients just two years ago!
Which Treatments are used for Melanoma?
Based on the stage of the cancer and other factors, your treatment options might include:
Early melanomas can be treated with surgery alone. More advanced cancers often require other treatments. Sometimes more than one type of treatment is used. To read about the most common ways of treating these cancers, see Treatment of Melanoma Skin Cancer by Stage.
Which Doctors treat Melanoma?
Depending on your treatment, you may have different types of doctors on your treatment team. These doctors may include:
- A dermatologist: a doctor who treats diseases of the skin
- A plastic surgeon (or reconstructive surgeon): a doctor who uses surgery to treat cancer and is able to reconstruct at the same time
- A medical oncologist: a doctor who treats cancer with medicines such as chemotherapy, immunotherapy, or targeted therapy
- A radiation oncologist: a doctor who treats cancer with radiation therapy
Many other specialists might be part of your treatment team as well, including physician assistants (PAs), nurse practitioners (NPs), nurses, nutrition specialists, social workers, and other health professionals. To learn more about who may be on your cancer care team, see Health Professionals Associated With Cancer Care.
Making Treatment Decisions
It’s important to discuss all of your treatment options as well as their possible side effects with your treatment team to help make the decision that best fits your needs. Some important things to think about include:
- Your age and health issues they may limit your ability to tolerate surgery
- The stage of melanoma
- The likelihood that treatment will cure your cancer (or help in some other way)
- Your feelings about the possible side effects from treatment
You may feel the need to make a decision quickly, but it is important to give yourself time to absorb the information you have just learned. It’s also very important to ask questions if there is anything you are unsure of. See What Should You Ask Your Health Care Team About Melanoma Skin Cancer? for some questions to ask.

Getting a Second Opinion
If time allows, you may feel the need for a second opinion from another doctor or medical team. This can give you more information and help you feel more certain about the treatment plan you choose. If you feel you need a second opinion, ask your doctor for help.
Clinical Trails
Clinical trials are controlled research studies organized to get a closer look at promising new treatments. Clinical trials are avenues to innovative cancer treatment
and sometimes the only way to access newer treatments. They help doctors to learn better protocols to treat cancer. There is always risk to new treatment, but the
rules require you to be well informed. Your oncologist can usually share insights into these new treatments under study.
If you would like to learn more about clinical trials that might be right for you, start by asking your doctor if your clinic or hospital conducts clinical trials. See Clinical Trials to learn more.
Considering Complementary and Alternative Methods
You may hear about alternative or complementary methods that your doctor hasn’t mentioned to treat your cancer or relieve symptoms. These methods can include vitamins, herbs, and special diets, or other methods such as acupuncture or massage, to name a few.
Complementary methods refer to treatments that are used along with your regular medical care. Alternative treatments are used instead of a doctor’s medical treatment. Although some of these methods might be helpful in relieving symptoms or helping you feel better, many have not been proven to work. Some might even be dangerous.
Be sure to talk to your cancer care team about any method you are thinking about using. They can help you learn what is known (or not known) about the method, which can help you make an informed decision. See Complementary and Alternative Medicine to learn more.
Your Choice for no Treatment at all
For some people, when treatments have been employed and are no longer controlling the cancer, it could be time to weigh the benefits and risks of continuing to try innovative treatments. Whether or not you choose to continue treatment, there are still things you can do to help augment your remaining quality of life. Learn more in If Cancer Treatments Stop Working.
Some people, especially if the cancer is advanced, might wish not to be treated at all. There are many reasons you might decide against cancer treatment, but it’s important to consult with your doctors before you make this decision. Remember that even if you choose against treating the cancer, you can still get supportive care to help with pain or other symptoms.
Again, developing melanoma in Phoenix is not uncommon and nothing to be ashamed of. Please seek help if you think you need it and feel free to contact us if you have any questions or would like to schedule an appointment.
Help getting through Cancer Treatment
Your cancer care team will be your first source of information and support, but there are other resources for help when you need it. Hospital- or clinic-based support services can be an important part of your care. These might include nursing or social work services, financial aid, nutritional advice, rehab, or spiritual help.
The American Cancer Society also has programs and services – including transportation to treatment, lodging, and more – to help you tolerate the treatment. Call our National Cancer Information Center at 1-800-227-2345 and speak with one of our trained specialists.
Melanoma Treatment Options
Surgery is the mainstay of therapy for early stage melanoma and for the resection of an isolated metastatic melanoma site. There are several different types of surgeries that are regularly performed to treat melanoma.
Radiation therapy uses high energy rays, like x-rays, to kill cancer cells. Although radiation is not an overly common melanoma treatment option, it is most often used as a symptom-relieving therapy in patients whose melanoma has spread to the brain or bones.
Clinical trials are research studies to test promising new or experimental cancer treatments. There are hundreds of clinical trials happening at any given time, and most experts agree that for a late-stage diagnosis, clinical trials are the best treatment option. You can also visit our Clinical Trial Finder to learn about clinical trials that are available.
Immunotherapy is a type of systemic therapy used in the treatment of melanoma at high risk for recurrence and metastases. The following immunotherapies are approved by the FDA for the treatment of melanoma: Imlygic (TVEC), Yervoy+Opdivo, Opdivo (nivolumab), Keytruda (pembrolizumab), Yervoy (ipilimumab),
Interleukin-2 (IL-2) and Interferon alpha 2-b.
Targeted therapy is a type of therapy where drugs (or other substances) “target” the abnormal aspects of tumor cells without harming normal cells. Several targeted therapies have been approved for use in treating various cancers, and this approach is now being evaluated in melanoma. You can also use a Targeted Therapy Finder to learn about possible treatment options for you. The following targeted therapies are approved by the FDA for the treatment of melanoma that is positive for the BRAF mutation: Zelboraf + Cotellic (Cobimetinib) , Tafinlar + Mekinist, Tafinlar (dabrafenib), Mekinist (trametinib) and Zelboraf (vemurafenib).
Chemotherapy is a type of systemic therapy intended to destroy melanoma cells throughout the body. Chemotherapy has shown limited success in the treatment of melanoma. Dacarbazine (DTIC) is FDA-approved for Stage IV (metastatic) melanoma.
Side Effects of Treatment
As with any cancer treatment, you or your loved one may experience side effects from the treatment regimen. With that in mind, it is important to remember that everyone reacts differently to treatment and experiences side effects in different ways. It is important that all side effects be reported to your treatment team AS SOON AS YOU BEGIN EXPERIENCING THEM.
The most common side effects of melanoma treatment include, but are not limited to:
- Diarrhea
- Vitiligo (loss of pigment)
- Skin rash
- Lymphedema
- Thyroid issues
- Colitis
- Fatigue
- Nausea
Melanoma Facts and Stats
Melanoma diagnoses are increasing at epidemic rates. You can help make a difference by knowing and sharing the facts about melanoma.
- Melanoma is the deadliest form of skin cancer. Skin cancer is the most common form of cancer in the U.S.
- Every hour of every day one American dies from melanoma – that’s approximately 10,000 per year.
- In 2018, over 178,000 Americans are expected to be diagnosed with melanoma. Of these, approximately, 91,000 will be diagnosed with invasive (Stage I, II, III or IV)melanoma and another 87,000 will be diagnosed with melanoma in situ(Stage 0).
- Melanoma is not just skin cancer. It can develop anywhere on the body – eyes, scalp, nails, feet, mouth, etc.
- Melanoma does not discriminate by age, race or gender.
- Melanoma is the leading cause of cancer death in young women ages 25-30 and the second leading cause of cancer death in women ages 30-35.
- In ages 15-29, melanoma is the second most commonly diagnosed cancer.
- The incidence of people under 30 developing melanomas is increasing faster than any other demographic group, soaring by 50% in women since 1980.
- Approximately 500 American childrenare diagnosed with melanoma each year.
- The majority of people diagnosed with melanoma are white men over the age of 50.
- Today, nearly 1 million people live with melanoma in the U.S.
- The lifetime risk of getting melanoma is about 1 in 40 for Caucasians, 1 in 200 for Hispanics and 1 in 1,000 for African Americans.
- Ocular melanoma, or melanoma of the eye, is the most common primary eye tumor in adults with around 2,000 new cases diagnosed each year in the United States.
- Mucosal melanomais a rare form of melanoma that develops in the sinuses, nasal passages, oral cavity, vagina, anus and other areas, making up about 1% of melanoma cases.
Melanoma Prevention Facts
- Nearly 90% of melanomas are thought to be caused by exposure to UV light and sunlight.
- It takes only one blistering sunburn, especially at a young age, to more than double a person’s chance of developing melanoma later in life.
- You can help prevent melanomaby seeking shade whenever possible, wearing protective clothing, avoiding direct sunlight between 10am-4pm and using broad spectrum sunscreen with SPF of at least 30 every day.
Indoor & Intentional Tanning Facts
- Indoor tanning devices are proven to cause cancer and have been classified into the highest cancer risk category by the World Health Organization’s International Agency for Cancer Research (IARC).
- Exposure to tanning beds before age 30 increases a person’s risk of developing melanoma by 75%.
- Young people who regularly use tanning beds are 8 times more likely to develop melanoma than people who have never used them.
- Research has found that indoor tanningdoes not protect against sunburn.
- Having 5 or more blistering sunburns early in life increases one’s melanoma risk by 80%.
For additional information, please visit the Melanoma Research Foundation website
There is an enormous amount of useful comforting information here to help you and your family through this disturbing experience.
Below are detailed scientific explanations of innovative target therapies and immunotherapies for melanoma that can be found on this link below. These are too detailed for most but are copied for your reference to this melanoma informational site:
Please visit this website for technical information and references.
https://www.skincancer.org/skin-cancer-information/melanoma/melanoma-treatments/advanced-treatment
- Some of the most important recent advances in melanoma treatment are increasingly effective new forms of immunotherapy. Using synthetic, mass-produced versions of natural immune system proteins, or by inhibiting proteins that block normal immune functions, immunotherapies boost the body’s ability to fight disease. New agents are adding years to many patients’ lives.
Checkpoint Blockade Therapy
The most successful form of melanoma immunotherapy to date is checkpoint blockade therapy, which now boasts four treatments approved by the FDA for stage IV, metastatic melanoma patients since 2011 — three individual drugs and a combined drug. Two of the individual drugs have also been approved for stage III patients who have metastases to their local lymph nodes. These treatments are significantly extending lives for many advanced (stages III and IV) melanoma patients. The medicines, all injected intravenously, have produced a bona fide revolution in melanoma treatment the past several years, giving real hope to patients.
The first successful checkpoint blockade therapy was ipilimumab (Yervoy®), approved in 2011 for patients with stage IV melanoma. Ipilimumab blocks CTLA-4 (cytotoxic T lymphocyte-associated protein 4), a protein receptor that functions as an immune checkpoint or “brake” to regulate the immune system. It can inhibit activation of T cells, thereby preventing them from destroying the tumor. By blocking CTLA-4, ipilimumab allows more T cells to be produced when needed to fight a cancer. It is often referred to as anti-CTLA-4 therapy.
A monoclonal antibody (a purified class of antibodies cloned and mass-produced in the lab from one specific type of cell or cell line), ipilimumab has yielded dramatic, sustained responses akin to “cures” in certain patients, with some surviving more than five or even 10 years. In a study of 1,861 patients treated with ipilimumab, about 22 percent lived three years or longer, and 84 percent of those survivors were estimated to be alive after five years and 10 years. One report, in fact, suggested that 20 percent of patients who received ipilimumab are alive after eight years. In contrast, only about 4 to 6 percent of patients on an earlier form of immunotherapy, interleukin-2, achieved long-term survival, and chemotherapy has never demonstrated a survival advantage.
In 2014, the FDA approved two additional immune checkpoint-blockading drugs, pembrolizumab (Keytruda®) and nivolumab (Opdivo®). Both drugs inhibit another molecule (programmed death-1, or PD-1) that suppresses T cells. PD-1 can directly interact with tumor cells by binding to a molecule called programmed death ligand-1 (PD-L1), and cancer cells may use PD-L1 to hide from attack by T cells. But the anti-PD-1 drugs can release the T cells to fight the cancer.
Both pembrolizumab and nivolumab were approved for use in patients whose melanoma has metastasized or cannot be removed by surgery. As of 2016, based on results showing they are more effective than ipilimumab or other therapies, both pembrolizumab and nivolumab can be used as frontline treatments — before other drug treatments have been tried — or as second-line treatments after other treatments have failed or stopped working. Studies have also shown that both nivolumab and pembrolizumab are safer than ipilimumab, with fewer serious side effects, produce greater response rates and are significantly more effective in fighting off melanoma. Both lead to longer periods without the disease advancing and longer survival. These agents have thus largely replaced ipilimumab as preferred options for frontline treatment, with ipilimumab still being used as a second-line therapy, as well as in conjunction with PD-1 inhibitors or other treatments.
A 2016 study of 655 patients on pembrolizumab offered some of the most impressive results ever with advanced melanoma patients. Average survival was 23 months, and more than 40 percent of patients — whether they had been previously treated with other drugs or not — were alive three years after starting treatment, with 85 patients remaining cancer-free. The fact that the therapy worked as well for previously treated patients as for previously untreated patients is especially good news for those whose initial drug therapies have failed to work or stopped working.
Nivolumab has produced comparable results. A 2016 report on the longest ongoing study of the drug shows 17-month average survival, with 42 percent of patients surviving three years and 34 percent surviving five.
Other PD-1 inhibitors for advanced melanoma such as pidilizumab (CT-011)are being tested, along with the related experimental inhibitors MPDL3280A (atezolizumab) and BMS-936559, which block PD-L1, the ligand that binds PD-1 to T cells and deactivates them. By blocking PD-L1, these inhibitory drugs keep it from interacting with PD-1, and thus release the T cells to fight the melanoma.
In late 2015, the FDA approved use of ipilimumab at an earlier stage, for stage III patients after their tumor and local lymph nodes are removed. This was the first time a checkpoint blockade therapy was used as an adjuvanttherapy — a supplementary treatment that enhances the effectiveness of a primary treatment such as surgery, and is designed to prevent or slow recurrence and possibly prevent metastasis beyond the lymph nodes. It was also the first therapy to improve significantly upon what was once the sole existing adjuvant immunotherapy for melanoma, high-dose interferon alfa-2b (IFN-alfa-2b).
The FDA based its acceptance of adjuvant ipilimumab on results from a phase 3 trial showing a 25 percent improvement in delaying recurrence in stage III patients treated with ipilimumab vs. placebo (27.6 months on average before the disease came back in the ipilimumab patients, vs. 17.1 months in the placebo patients). This is somewhat longer than achieved with IFN-alpha-2b.
Longer-term follow-up of the study has produced even more important results. In October, 2016, researchers for the European Organization for Research and Treatment of Cancer (EORTC) published findings in the New England Journal of Medicine demonstrating that ipilimumab increased survival in these patients, reducing risk of death by 28 percent compared to placebo treatment. Five-year survival (overall survival, or OS) rates were 65.4 percent for patients in the ipilimumab group vs. 54.4 percent for those in the placebo group. All patients had previously gone through surgery to remove their primary tumors. This made ipilimumab the first proven life-extending treatment for stage III melanoma patients in history.
In December 2017, the FDA also approved the use of nivolumab as an adjuvant therapy, for stage III patients with lymph node metastases whose primary tumors have been completely removed, as well as stage IV patients with distant metastatic disease.
The research leading to this approval showed greater than 66 percent recurrence-free survival (RFS) rate at 18 months with nivolumab, compared with 52 percent RFS with ipilimumab. It also showed a 35 percent reduction in the risk of recurrence or death with nivolumab compared with ipilimumab. Furthermore, serious side effects were significantly lower with nivolumab than with ipilimumab (about 14 percent of cases versus 46 percent).
Combined Checkpoint Blockade Therapies
Building on the success of the individual checkpoint blockade therapies, investigators have pursued different avenues in combining them to even greater effect. In 2015, the FDA approved one such combination therapy, nivolumab-ipilimumab, for patients with metastatic or inoperable melanoma, and findings to date show it is indeed more effective than either ipilimumab or nivolumab alone. The ongoing phase 3 CheckMate-067 trial has found that the combination reduces disease progression by about 58 percent in previously untreated patients, compared with 45 percent reduction with nivolumab alone and 19 percent with ipilimumab alone. About 17 percent of patients on the combination therapy appear to have gone into complete remission. Of the initial 53 patients treated with the combination therapy, an astonishing 68 percent have survived three years or longer.
One thing to be aware of is that patients on the combination therapy have a higher percentage of side effects and complications, such as thyroid or kidney damage, that can be serious enough to discontinue the therapy. However, some investigators suggest that for patients who have such serious reactions — even if they have to be hospitalized and go on steroids for weeks or months to recover from the side effects — the ultimate life-extending benefits may be worth the risk of using the combination therapy rather than the single-agent therapies. Much of this depends on the age, health and hardiness of the patient. Until longer follow-up and survival data emerge, choosing between a solo therapy or the combination therapy will remain an important decision for doctors and patients.
Oncolytic Virus Injections
In late 2015, the FDA approved the first drug in an entirely new class of immunotherapies for melanoma: injectable oncolytic virus therapy. The drug, talimogene laherparepvec (Imlygic®), often shortened to T-VEC, is approved for local treatment of inoperable metastatic lesions in the skin or lymph nodes that recur after initial surgery.
An oncolytic virus is one designed to specifically target, infect and kill cancer cells. Imlygic is a version of the herpes simplex virus genetically modified to select cancer cells but not healthy cells for infection, while also secreting an immune-boosting protein added to the drug that can strengthen the body’s immune response against melanoma. Over many months, a massive amount of the virus is injected directly into detectable skin tumors, where it replicates inside the tumor cells, causing them to rupture and die. At the same time, the immune-boosting protein in the drug kick-starts the immune system to attack the injected tumors.
The question remains whether the immune system attacks only the injected tumors or goes on to attack tumors throughout the body, but in the clinical trials, the therapy increased durable response rates (up to six months) for patients with advanced disease, shrinking tumors in about 16 percent of patients. While these benefits are fairly limited, and while the drug has not proven to extend survival or prevent metastases to the organs, T-VEC represents a new avenue for treatment that researchers hope to improve upon significantly. This agent is also being tested in combination with other therapies.
Similar oncolytic viruses are now being tested, with the goal of producing more dramatic results for melanoma patients who have recurrences.
Adoptive Cell Transfer
The use of white blood cells called tumor-infiltrating lymphocytes (TILs) is another, still experimental, avenue for immunotherapy in advanced melanoma patients. Of special note is a technique from the National Cancer Institute called adoptive cell transfer (ACT), which involves harvesting TILs from the patient’s blood. Scientists identify and isolate the most effective melanoma-killing T cells from these TILs. They then grow them in large numbers in the lab and reinject them into the patient in the hope that they will massively attack the patient’s melanoma cells. The doctors may add high doses of the immunotherapy interleukin-2 to make these tumor-fighting cells mature and multiply, and may also use certain drugs to eliminate immune factors (and even bone marrow) that might inhibit the tumor-fighting cells. (This is called lymphodepletion.) In clinical trials with metastatic melanoma patients who had not responded to previous treatment, the patients’ response rates have been far higher than those seen with chemotherapy.
In the latest trials, total-body irradiation was added to enhance lymphodepletion, and response rates up to 72 percent were observed in 93 patients, with 11 achieving complete remissions lasting 18 to 75 months or more.
Reduced Roles for Earlier Immunotherapies
As recently as five years ago, injectable interferon alfa-2b for adjuvant use (intended to prevent recurrence or metastatic spread) in high-risk stage II and stage III patients and interleukin-2 (for stage IV patients) were the only approved immunotherapies for advanced melanoma. However, adjuvant interferon in low-, intermediate- and high-dose regimens (the high-dose regimen is the only one approved in the U.S.) has proven to have little or no effect on patients’ survival, and interleukin-2 has improved survival for an extremely limited percentage of patients. With the success of the revolutionary checkpoint blockade immunotherapies and targeted therapies, high-dose interferon and interleukin-2 have been relegated to backup or complementary roles used after other therapies, to reinforce other therapies or as a treatment arm in clinical trials testing other drugs.
In 2011, the FDA approved a new variation of high-dose interferon alfa-2b called peginterferon alfa-2b (Sylatron®) as an adjuvant therapy to treat stage III melanoma patients, but it has not been demonstrated to improve patients’ length of survival either.
The advances in understanding melanoma and the immune system have set the stage for continual improvements in the treatment of advanced disease. A substantial proportion of patients have already derived significant long-term benefits and are now living years longer than in the past.
The next goal will be to determine which combinations and methods are most suitable to shrink melanoma most effectively, maintain the best possible quality of life for patients and extend patients’ lives as long as possible. Many other novel approaches are also on the horizon, currently either in active laboratory study or clinical trials; the hope is to turn metastatic melanoma from a deadly disease into a manageable chronic condition.
When melanoma cells spread to the lymph nodes (stage III) or more distant parts of the body (stage IV), including organs, the disease is considered advanced, and additional therapy usually follows surgical removal of the original (primary) skin tumor. (This may also be the case with stage II patients whose melanomas are considered at high risk of spreading to the lymph nodes or beyond.) Today, patients with metastatic melanoma can benefit from an array of treatments approved by the FDA in recent years that can extend their lives by months or years, with a rising number of patients going into long-term remission. With several effective treatments available, the decisions patients and their physicians make about which treatments to use are more important than ever, a vital part of the treatment process. A variety of factors may influence this decision.
Below, you’ll find the key treatments now available for advanced melanoma patients. Ask your physician to explain the possibilities and the reasons for selecting one treatment over another. For more extended definitions of individual treatments, please refer to our Skin Cancer Treatment Glossary.
Select a treatment to learn more about it.
- Some of the most important recent advances in melanoma treatment are increasingly effective new forms of immunotherapy. Using synthetic, mass-produced versions of natural immune system proteins, or by inhibiting proteins that block normal immune functions, immunotherapies boost the body’s ability to fight disease. New agents are adding years to many patients’ lives.
Checkpoint Blockade Therapy
The most successful form of melanoma immunotherapy to date is checkpoint blockade therapy, which now boasts four treatments approved by the FDA for stage IV, metastatic melanoma patients since 2011 — three individual drugs and a combined drug. Two of the individual drugs have also been approved for stage III patients who have metastases to their local lymph nodes. These treatments are significantly extending lives for many advanced (stages III and IV) melanoma patients. The medicines, all injected intravenously, have produced a bona fide revolution in melanoma treatment the past several years, giving real hope to patients.
The first successful checkpoint blockade therapy was ipilimumab (Yervoy®), approved in 2011 for patients with stage IV melanoma. Ipilimumab blocks CTLA-4 (cytotoxic T lymphocyte-associated protein 4), a protein receptor that functions as an immune checkpoint or “brake” to regulate the immune system. It can inhibit activation of T cells, thereby preventing them from destroying the tumor. By blocking CTLA-4, ipilimumab allows more T cells to be produced when needed to fight a cancer. It is often referred to as anti-CTLA-4 therapy.
A monoclonal antibody (a purified class of antibodies cloned and mass-produced in the lab from one specific type of cell or cell line), ipilimumab has yielded dramatic, sustained responses akin to “cures” in certain patients, with some surviving more than five or even 10 years. In a study of 1,861 patients treated with ipilimumab, about 22 percent lived three years or longer, and 84 percent of those survivors were estimated to be alive after five years and 10 years. One report, in fact, suggested that 20 percent of patients who received ipilimumab are alive after eight years. In contrast, only about 4 to 6 percent of patients on an earlier form of immunotherapy, interleukin-2, achieved long-term survival, and chemotherapy has never demonstrated a survival advantage.
In 2014, the FDA approved two additional immune checkpoint-blockading drugs, pembrolizumab (Keytruda®) and nivolumab (Opdivo®). Both drugs inhibit another molecule (programmed death-1, or PD-1) that suppresses T cells. PD-1 can directly interact with tumor cells by binding to a molecule called programmed death ligand-1 (PD-L1), and cancer cells may use PD-L1 to hide from attack by T cells. But the anti-PD-1 drugs can release the T cells to fight the cancer.
Both pembrolizumab and nivolumab were approved for use in patients whose melanoma has metastasized or cannot be removed by surgery. As of 2016, based on results showing they are more effective than ipilimumab or other therapies, both pembrolizumab and nivolumab can be used as frontline treatments — before other drug treatments have been tried — or as second-line treatments after other treatments have failed or stopped working. Studies have also shown that both nivolumab and pembrolizumab are safer than ipilimumab, with fewer serious side effects, produce greater response rates and are significantly more effective in fighting off melanoma. Both lead to longer periods without the disease advancing and longer survival. These agents have thus largely replaced ipilimumab as preferred options for frontline treatment, with ipilimumab still being used as a second-line therapy, as well as in conjunction with PD-1 inhibitors or other treatments.
A 2016 study of 655 patients on pembrolizumab offered some of the most impressive results ever with advanced melanoma patients. Average survival was 23 months, and more than 40 percent of patients — whether they had been previously treated with other drugs or not — were alive three years after starting treatment, with 85 patients remaining cancer-free. The fact that the therapy worked as well for previously treated patients as for previously untreated patients is especially good news for those whose initial drug therapies have failed to work or stopped working.
Nivolumab has produced comparable results. A 2016 report on the longest ongoing study of the drug shows 17-month average survival, with 42 percent of patients surviving three years and 34 percent surviving five.
Other PD-1 inhibitors for advanced melanoma such as pidilizumab (CT-011)are being tested, along with the related experimental inhibitors MPDL3280A (atezolizumab) and BMS-936559, which block PD-L1, the ligand that binds PD-1 to T cells and deactivates them. By blocking PD-L1, these inhibitory drugs keep it from interacting with PD-1, and thus release the T cells to fight the melanoma.
In late 2015, the FDA approved use of ipilimumab at an earlier stage, for stage III patients after their tumor and local lymph nodes are removed. This was the first time a checkpoint blockade therapy was used as an adjuvanttherapy — a supplementary treatment that enhances the effectiveness of a primary treatment such as surgery, and is designed to prevent or slow recurrence and possibly prevent metastasis beyond the lymph nodes. It was also the first therapy to improve significantly upon what was once the sole existing adjuvant immunotherapy for melanoma, high-dose interferon alfa-2b (IFN-alfa-2b).
The FDA based its acceptance of adjuvant ipilimumab on results from a phase 3 trial showing a 25 percent improvement in delaying recurrence in stage III patients treated with ipilimumab vs. placebo (27.6 months on average before the disease came back in the ipilimumab patients, vs. 17.1 months in the placebo patients). This is somewhat longer than achieved with IFN-alpha-2b.
Longer-term follow-up of the study has produced even more important results. In October, 2016, researchers for the European Organization for Research and Treatment of Cancer (EORTC) published findings in the New England Journal of Medicine demonstrating that ipilimumab increased survival in these patients, reducing risk of death by 28 percent compared to placebo treatment. Five-year survival (overall survival, or OS) rates were 65.4 percent for patients in the ipilimumab group vs. 54.4 percent for those in the placebo group. All patients had previously gone through surgery to remove their primary tumors. This made ipilimumab the first proven life-extending treatment for stage III melanoma patients in history.
In December 2017, the FDA also approved the use of nivolumab as an adjuvant therapy, for stage III patients with lymph node metastases whose primary tumors have been completely removed, as well as stage IV patients with distant metastatic disease.
The research leading to this approval showed greater than 66 percent recurrence-free survival (RFS) rate at 18 months with nivolumab, compared with 52 percent RFS with ipilimumab. It also showed a 35 percent reduction in the risk of recurrence or death with nivolumab compared with ipilimumab. Furthermore, serious side effects were significantly lower with nivolumab than with ipilimumab (about 14 percent of cases versus 46 percent).
Combined Checkpoint Blockade Therapies
Building on the success of the individual checkpoint blockade therapies, investigators have pursued different avenues in combining them to even greater effect. In 2015, the FDA approved one such combination therapy, nivolumab-ipilimumab, for patients with metastatic or inoperable melanoma, and findings to date show it is indeed more effective than either ipilimumab or nivolumab alone. The ongoing phase 3 CheckMate-067 trial has found that the combination reduces disease progression by about 58 percent in previously untreated patients, compared with 45 percent reduction with nivolumab alone and 19 percent with ipilimumab alone. About 17 percent of patients on the combination therapy appear to have gone into complete remission. Of the initial 53 patients treated with the combination therapy, an astonishing 68 percent have survived three years or longer.
One thing to be aware of is that patients on the combination therapy have a higher percentage of side effects and complications, such as thyroid or kidney damage, that can be serious enough to discontinue the therapy. However, some investigators suggest that for patients who have such serious reactions — even if they have to be hospitalized and go on steroids for weeks or months to recover from the side effects — the ultimate life-extending benefits may be worth the risk of using the combination therapy rather than the single-agent therapies. Much of this depends on the age, health and hardiness of the patient. Until longer follow-up and survival data emerge, choosing between a solo therapy or the combination therapy will remain an important decision for doctors and patients.
Oncolytic Virus Injections
In late 2015, the FDA approved the first drug in an entirely new class of immunotherapies for melanoma: injectable oncolytic virus therapy. The drug, talimogene laherparepvec (Imlygic®), often shortened to T-VEC, is approved for local treatment of inoperable metastatic lesions in the skin or lymph nodes that recur after initial surgery.
An oncolytic virus is one designed to specifically target, infect and kill cancer cells. Imlygic is a version of the herpes simplex virus genetically modified to select cancer cells but not healthy cells for infection, while also secreting an immune-boosting protein added to the drug that can strengthen the body’s immune response against melanoma. Over many months, a massive amount of the virus is injected directly into detectable skin tumors, where it replicates inside the tumor cells, causing them to rupture and die. At the same time, the immune-boosting protein in the drug kick-starts the immune system to attack the injected tumors.
The question remains whether the immune system attacks only the injected tumors or goes on to attack tumors throughout the body, but in the clinical trials, the therapy increased durable response rates (up to six months) for patients with advanced disease, shrinking tumors in about 16 percent of patients. While these benefits are fairly limited, and while the drug has not proven to extend survival or prevent metastases to the organs, T-VEC represents a new avenue for treatment that researchers hope to improve upon significantly. This agent is also being tested in combination with other therapies.
Similar oncolytic viruses are now being tested, with the goal of producing more dramatic results for melanoma patients who have recurrences.
Adoptive Cell Transfer
The use of white blood cells called tumor-infiltrating lymphocytes (TILs) is another, still experimental, avenue for immunotherapy in advanced melanoma patients. Of special note is a technique from the National Cancer Institute called adoptive cell transfer (ACT), which involves harvesting TILs from the patient’s blood. Scientists identify and isolate the most effective melanoma-killing T cells from these TILs. They then grow them in large numbers in the lab and reinject them into the patient in the hope that they will massively attack the patient’s melanoma cells. The doctors may add high doses of the immunotherapy interleukin-2 to make these tumor-fighting cells mature and multiply, and may also use certain drugs to eliminate immune factors (and even bone marrow) that might inhibit the tumor-fighting cells. (This is called lymphodepletion.) In clinical trials with metastatic melanoma patients who had not responded to previous treatment, the patients’ response rates have been far higher than those seen with chemotherapy.
In the latest trials, total-body irradiation was added to enhance lymphodepletion, and response rates up to 72 percent were observed in 93 patients, with 11 achieving complete remissions lasting 18 to 75 months or more.
Reduced Roles for Earlier Immunotherapies
As recently as five years ago, injectable interferon alfa-2b for adjuvant use (intended to prevent recurrence or metastatic spread) in high-risk stage II and stage III patients and interleukin-2 (for stage IV patients) were the only approved immunotherapies for advanced melanoma. However, adjuvant interferon in low-, intermediate- and high-dose regimens (the high-dose regimen is the only one approved in the U.S.) has proven to have little or no effect on patients’ survival, and interleukin-2 has improved survival for an extremely limited percentage of patients. With the success of the revolutionary checkpoint blockade immunotherapies and targeted therapies, high-dose interferon and interleukin-2 have been relegated to backup or complementary roles used after other therapies, to reinforce other therapies or as a treatment arm in clinical trials testing other drugs.
In 2011, the FDA approved a new variation of high-dose interferon alfa-2b called peginterferon alfa-2b (Sylatron®) as an adjuvant therapy to treat stage III melanoma patients, but it has not been demonstrated to improve patients’ length of survival either.
- Targeted therapies, among the most revolutionary treatments for advanced melanoma, use drugs or other substances to identify and attack specific types of cancer cells, or to block the action of certain genes, enzymes, proteins or other molecules that promote the growth and spread of cancer cells. This allows the cancerous cells to be treated without killing healthy cells.
In the past decade, there have been several notable successes in targeted melanoma therapy. The first was vemurafenib (Zelboraf®), FDA-approved in 2011, a drug taken by mouth that inhibits a defective (mutated) version of a gene called BRAF. BRAF produces a protein that normally regulates skin cells, causing them to multiply only when growth is needed. However, specific mutations in BRAF called v600E (found in about half of all melanoma patients), and less frequently, two other defective versions of BRAF called v600K and v600D, produce an abnormal version of the protein that stays switched on. This leads to out-of-control cellular growth, i.e., cancer. Vemurafenib can bind to the defective protein and deactivate it. Studies have shown that it produces rapid, striking antitumor activity in patients with BRAF V600E- and V600K-mutated melanoma, delaying disease progression and increasing patients’ survival compared with standard chemotherapy (median 13.6 months’ survival for vemurafenib patients vs. 9.7 months for chemotherapy patients). And some patients go much longer before recurrence. However, most patients eventually develop resistance to the treatment, and the melanoma starts to grow and advance again.
In hopes of delaying resistance and increasing survival, scientists developed two other targeted treatments, one directed at BRAF and another targeting a related molecule called MEK. The FDA approved the BRAF inhibitor, dabrafenib (Taflinar®), and the MEK inhibitor, trametinib (Mekinist®), both taken by mouth, in 2013. Like vemurafenib, these newer targeted therapies can be used only in patients who have the defective BRAF gene.
Used in combination, dabrafenib and trametinib produce more rapid responses and higher response rates in metastatic melanoma patients than either agent or vemurafenib alone. The idea behind this combination therapy is that even when the BRAF inhibitor meets with resistance in inhibiting melanoma, the MEK inhibitor further slows progression of the disease by blocking MEK, at least delaying the melanoma’s advance.
In 2014, the FDA approved the use of combination dabrafenib and trametinib for patients who have inoperable or metastatic melanoma with a BRAF V600E or V600K mutation. Due to its superior results, the combination therapy is now generally used in preference to vemurafenib, dabrafenib or trametinib alone. This drug combination has reduced resistance, increased tumor shrinkage and extended the length of time before the melanoma starts growing again, leading to longer survival. The latest studies show that a remarkable 51 percent of BRAF-mutated metastatic melanoma patients on combination dabrafenib-trametinib are still alive at two years, with median survival of 25.6 months, vs. 45 percent of patients on dabrafenib alone and 38 percent of patients on vemurafenib alone (median survival 18 months).
In 2015, the FDA approved a second targeted combination BRAF/MEK inhibitor therapy for advanced melanoma. Combining the established BRAF blocker vemurafenib with a new oral MEK blocker called cobimetinib (Cotellic®), the therapy was approved for stage IV BRAF-mutated patients with melanoma that is inoperable or has metastasized throughout the body. This new combination therapy works similarly to dabrafenib-trametinib. Like dabrafenib, vemurafenib blocks the mutant BRAF gene, while cobimetinib, like trametinib, blocks MEK. By inhibiting these two different parts of the signaling pathway that promotes metastasis, the new combination therapy delays advance of the disease about five months longer on average (12 months vs. seven months) than vemurafenib alone. Patients on this combination therapy also live longer on average than those on vemurafenib alone, with approximately 65 percent of patients alive 17 months after starting treatment, compared with about 50 percent of those taking vemurafenib only.
With both of the combination therapies, dabrafenib-trametinib and vemurafenib-cobimetinib, producing comparably superior results to vemurafenib or dabrafenib alone, they have become the standard frontline targeted options for treating BRAF-mutant melanoma, although single-drug therapy may still be useful in certain situations. In addition, the checkpoint blockade immunotherapies have become an alternative front-line choice for metastatic melanoma patients, even those with the defective BRAF gene.
In December 2017, the FDA granted a priority review designation to combination dabrafenib-trametinib as an adjuvant treatment for patients with BRAF V600E-positive or V600K-positive stage III melanoma following complete removal of the primary tumor. This status was conferred based on the COMBI-AD trial, the first randomized study ever of combination BRAF-MEK inhibition as a melanoma adjuvant therapy. In the study, the combination reduced the risk of disease recurrence or death by 53 percent compared with placebo for patients with BRAF-mutant stage III melanoma. After a median follow-up of 2.8 years, the three-year recurrence-free survival rate with dabrafenib-trametinib was 58 percent compared with 39 percent for the placebo arm. Early data on overall survival showed that 86 percent of patients in the combination arm were still alive at three years, versus 77 percent in the placebo arm.
Adjuvant therapies are strategies that enhance the effectiveness of a primary treatment such as surgery, with the goal of delaying recurrence and extending overall survival. The hope is that by using this medicine before the cancer reaches stage IV, spreading throughout the body, it will provide even greater benefits for patients and save more lives.
- With the rapid evolution of immunotherapies and targeted therapies for advanced melanoma in the past five years, the choice of frontline treatment has continually shifted. With FDA approval of the BRAF inhibitor vemurafenib (Zelboraf®)in 2011, targeted anti-BRAF therapy became the standard frontline treatment for advanced melanoma patients with the defective BRAF gene. For patients who did not have the defective gene, the checkpoint blockade immunotherapy ipilimumab (Yervoy®), also approved in 2011, became the frontline treatment.
Then, when the combination targeted therapy dabrafenib-trametinib was approved in 2013, proving more effective than either vemurafenib or dabrafenib alone, combination dabrafenib-trametinib came to be accepted as the preferable frontline treatment for patients with the mutant BRAF gene. Ipilimumab remained the first option for those who did not have the mutant gene, and the second-line therapy for patients in whom the targeted therapy failed or stopped working.
The field shifted again in 2014, when the new PD-1-inhibiting checkpoint blockade therapies pembrolizumab (Keytruda®) and nivolumab (Opdivo®)were approved. Studies soon showed them to be safer and more effective than ipilimumab, and they eventually replaced ipilimumab as the frontline therapy for advanced melanoma patients who did not have the mutant BRAF gene. Then, combination nivolumab-ipilimumab was approved in 2015, proving somewhat more effective than pembrolizumab, nivolumab or ipilimumab alone.
Later that year, the FDA approved the targeted combination therapy vemurafenib-cobimetinib (Cotellic®), comparable to the combination targeted therapy dabrafenib-trametinib, adding to patients’ available options.
Most recently, research has shown that the approved checkpoint blockade therapies pembrolizumab, nivolumab and combination nivolumab-ipilimumab are all effective whether or not patients have the mutant BRAF gene, and are often more effective than any of the targeted BRAF therapies.
Depending on their situation and personal preferences, advanced melanoma patients now most often choose from among five treatments as their frontline therapy: the single agent checkpoint blockade therapies nivolumab or pembrolizumab, combined nivolumab-ipilimumab or the targeted combination therapies dabrafenib-trametinib or vemurafenib-cobimetinib. A variety of factors may influence the decision. For example, the targeted therapies tend to begin working faster, often eliminating many or all tumors at a rapid pace. Thus, if the patient is in a desperate state and needs rapid elimination of tumors to survive, combination dabrafenib-trametinib or combination vemurafenib-cobimetinib might be the frontline choice for patients with the mutant BRAF gene.
However, this therapy most often begins to fail sooner than the immunotherapies, so if rapid elimination of tumors is not as important, the patient and physician might opt for one of the checkpoint blockade immunotherapies, which offer the best chance of long-term survival. The most effective of all these therapies at present is combination nivolumab-ipilimumab (by a small margin over pembrolizumab or nivolumab alone), which may make it the first choice, particularly if rapid response is needed in a patient with advanced melanoma. However, it is a riskier therapy than either pembrolizumab or nivolumab alone, with a greater chance of serious side effects that might stop the therapy, so for older or frailer patients with poorer immune systems, either pembrolizumab or nivolumab alone might still be the first choice.
Ultimately, of course, making these decisions remains up to you and your doctor, based on your health and other factors. The good news is that today, if your first therapy doesn’t work or stops working, other effective therapies now exist that often can be tried next. Researchers are continually homing in on which combinations, methods and sequences are most suitable to slow or eliminate melanoma most effectively, maintain the best possible quality of life for patients and extend their lives as long as possible. Many other new approaches are also on the horizon, currently in active laboratory study or clinical trials. The hope is to turn metastatic melanoma into a manageable or even curable condition.
- A number of chemotherapy drugs active in fighting cancer cells have been used to treat melanoma, either one at a time or in combination. However, with new immunotherapies and targeted therapies producing much better results, the chemotherapies have been phased out as frontline treatments. They are sometimes used to supplement the other treatments. To date, dacarbazine (DTIC), given by intravenous injection, is the only chemotherapy approved for melanoma by the U.S. Food and Drug Administration (FDA). DTIC may be combined with carmustin (BCNU) and tamoxifen, or with cisplatinand vinblastine. Temozolomide, an oral drug closely resembling DTIC, is FDA-approved for brain cancers but also used off-label (without specific FDA approval) for melanomas that have spread to the brain or nervous system.
- Radiation, directing high-energy X-ray beams at the cancer cells to destroy them, is being combined experimentally with some of the medications used for advanced melanoma, and results so far are promising. External beam radiation therapy (EBRT) focuses radiation from outside the body on the skin tumor.
While rarely used to treat a primary melanoma tumor, radiation therapy can also sometimes be used after surgery to lower the chance of recurrence in a site where lymph nodes have been removed, especially if many nodes were cancerous. In addition, it may be used on occasion to help treat melanoma that has come back after surgery in the skin or lymph nodes. Radiation therapy has a definite role in treating spread of melanoma to the brain and in relieving symptoms and pain in specific areas where the melanoma has already spread, by shrinking or slowing the tumor’s growth. Radiation therapy is now being studied in combination with immune checkpoint inhibitors to see if it can enhance the antitumor immune response.
- Many patients, especially those with advanced disease, participate in clinical trials to obtain new treatments that may be more effective than standard therapy but are still experimental and not generally available.
Patients who have stage III and IV melanoma might consider enrolling in a clinical trial of a new or experimental treatment. There are risks involved in enrolling in a clinical trial, but there can be benefits as well. Discuss the possibilities with your doctor. More treatment possibilities exist than ever before, giving ever greater hope to people with advanced melanoma.
The advances in understanding melanoma and the immune system have set the stage for continual improvements in the treatment of advanced disease. A substantial proportion of patients have already derived significant long-term benefits and are now living years longer than in the past.
The next goal will be to determine which combinations and methods are most suitable to shrink melanoma most effectively, maintain the best possible quality of life for patients and extend patients’ lives as long as possible. Many other novel approaches are also on the horizon, currently either in active laboratory study or clinical trials; the hope is to turn metastatic melanoma from a deadly disease into a manageable chronic condition.
Dr J and his staff look forward to understanding the details of your unique case, desires and aspirations, and to providing you with realistic, safe and attainable results that leave you looking beautiful, and truly feeling like yourself. Take the first step toward your healing or rejuvenating procedure with Dr J by filling out our contact form to request a consultation, or to inquire about any of the services we offer. We look forward to treating you!
[ultimate_spacer height=”30″]
Get started today!
Dr J and his staff are committed to providing you with exceptional and compassionate care. On behalf of our entire team, we invite you to request a consultation to talk to Dr J about your goals, expectations and aspirations. We can’t wait to find out how our 20-plus years of experience in cosmetic and functional plastic surgery can help change your life, and make you a happier, healthier person.
Dr. William Jacobsen
Plastic Surgery
2525 East Arizona
Biltmore Circle, Suite C236
Phoenix, AZ 85016
Phone: 602-212-0100
Fax: 602.279.1701
[email protected]
[ultimate_spacer height=”30″]
Our Procedures & Treatments
Dr J considers every surgery an opportunity to express his vision with his patients, and believes in natural, beautiful results that leave you feeling confident and beautiful, but most importantly, feeling like yourself. From extremely rare and complex surgical cases, to cosmetic surgery, Dr J has the experience, compassion and understanding to help you achieve your surgical goals.
[ultimate_spacer height=”30″]
Request an Appointment
[ultimate_spacer height=”10″]





